 |
MAGISKA MOLEKYLERS WIKI |
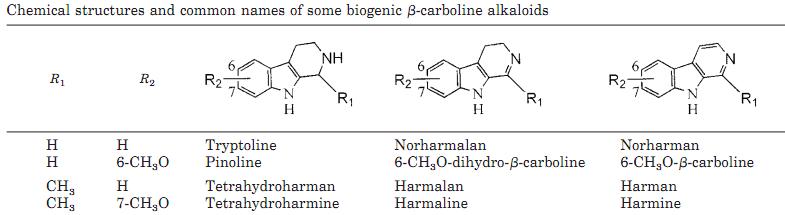
Harmelalkaloider
Inneh├źll
Generell information
Harmelalkaloider (├żven kallade harmala alkaloider) ├żr samlingsnamnet f├Čr alkaloiderna harmin, harmalin, harman, harmaol och liknande substanser med liknande molekylstruktur. Harmelalkaloider ing├źr i gruppen betakarboliner.
N├żr man unders├Čkte v├żxten Banisteriopsis caapi fann man ett nytt ├żmne som man kallade Telepathin. Senare efterforskningar visade dock att det redan uppt├żckts i Peganum harmala och kallades harmin.
Harmelalkaloider ├żr naturligt f├Črekommande MAO-h├żmmare. Det vanligaste anv├żndningsomr├źdet bland anv├żndare av psykedeliska droger ├żr f├Čr att g├Čra DMT aktivt vid oralt bruk i form av Ayahuasca eller f├Čr att f├Črst├żrka effekten av andra tryptaminer, men ├żven ensamma ger dom en hallucinogen effekt vid h├Čgre doser. Alexander Shulgin vittnar i Tihkal om att visuella effekter existerar i form av bl.a CEV:s. Ut├Čver det m├żrks ├żven en sederande effekt. Men i dessa h├Čgre doser ├żr ├żven bieffekterna mer framtr├żdande och g├Čr att harmelalkaloiderna ej ├żr eftertraktade som berusningsdroger.
Varning! Det kan vara farligt att kombinera MAO-h├żmmare och vissa typer av mat och droger. L├żs mer i artikeln MAO-h├żmmare.
Ursprungsbefolkningarna i Sydamerika som anv├żnder Ayahuasca tillskriver den st├Črre delen av den andliga och helande kraften till b. caapi och brukar anv├żnda mycket mindre DMT-v├żxter ├żn vad som blivit popul├żrt bland v├żsterl├żnningar.
Harmelalkaloiderna har ├żven anv├żnts f├Čr att avgifta narkomaner [1] och ett flertal vetenskapliga unders├Čkningar underst├Čdjer p├źst├źenden [2] [3] [4] [5] [6] [7]
Alkaloidhalt i olika v├żxter
| V├żxtens namn | Harmala |
|---|---|
| Peganum harmala | 2 - 7% |
| Banisteriopsis caapi | varierande 0,1 - 1,0% fr├żmst i l├żgre skalan. |
| Passiflora incarnata | 0,05 - 0,1% |
| Tribulus terrestris | Sv├źruppskattad |
K├żlla till de f├Črsta tre: [8]
F├Črklaring av ratio: F├Čr att exempelvis f├ź effekten av ett gram P. harmala m├źste 20 gram B. caapi anv├żndas.
V├żxter som inneh├źller harmelalkaloider
AGARICACEAE
- Coriolus maximus (Harman)
APOCYNACEAE
- Amsonia tabernaemontana (Harmin etc.)
- Apocynum cannabinum (Harmalol)
- Ochrosia nakaiana (Harman)
BIGNONIACEAE
- Newbouldia laevis (Harman)
CALYCANTHACEAE
- Calycanthus occidentalis (Harmin)
CHENOPODIACEAE
- Hammada leptoclada (Tetrahydroharman etc.)
- Kochia scoparia (Harmin etc.)
COMBRETACEAE
- Guiera senegalensis (Harman etc.)
- Carex brevicollis (Harmin etc.)
ELAEAGNACEAE
- Elaeagnus augustifolia (Harman etc.)
- Elaeagnus hortensis (Tetrahydroharman etc.)
- Elaeagnus orientalis (Tetrahydroharman)
- Elaeagnus spinosa (Tetrahydroharman)
- Hippophae rhammoides (Harman etc.)
- Shepherdia argentea (Tetrahydroharmol)
- Shepherdia canadensis (Tetrahydroharmol)
GRAMINEAE
- Arundo donax (Tetrahydroharman)
- Festuca arundinacea (Harman etc.)
- Lolium perenne (Harman etc.)
- Acacia baileyana (Tetrahydroharman)
- Acacia complanata (Tetrahydroharman etc.)
- Burkea africana (Harman etc.)
- Desmodium pulchellum (Harman etc.)
- Mucuna pruriens (6-methoxy-harman)
- Petalostylis labicheoides (Tetrahydroharman)
- Prosopis nigra (Harman etc.)
LOGANIACEAE
- Strychnos usambarensis (Harman)
MALPIGHIACEAE
- Banisteriopsis caapi (Harmin, tetrahydroharmin, harmalin etc.)
- Banisteriopsis lutea (Harmin)
- Banisteriopsis muricata (Harmin)
- Callaeum antifebrile (= Cabe paraensis) (Harmin)
MYRISTICACEAE
- Virola cuspidata (6-Methoxy-Harman)
PASSIFLORACEAE
Sl├żktet Passiflora:
- Passiflora actinea (Harman = Passiflorin)
- Passiflora alata (Harman)
- Passiflora alba (Harman)
- Passiflora bryonoides (Harman)
- Passiflora caerulea (Harman)
- Passiflora capsularis (Harman)
- Passiflora decaisneana (Harman)
- Passiflora edulis (Harman)
- Passiflora eichleriana (Harman)
- Passiflora foetida (Harman)
- Passiflora incarnata (Harmin, harmalin, harman, etc.)
- Passiflora quadrangularis (Harman)
- Passiflora ruberosa (Harman)
- Passiflora subpeltata (Harman)
- Passiflora warmingii (Harman)
POLYGONACEAE
- Leptactinia densiflora (Leptaflorin, etc.)
- Nauclea diderrichii (Harman etc.)
- Ophiorrhiza japonica (Harman)
- Pauridiantha callicarpoides (Harman)
- Pauridiantha dewevrei (Harman)
- Pauridiantha lyalli (Harman)
- Pauridiantha viridiflora (Harman)
- Simira klugii (Harman)
- Simira rubra (Harman)
- Uncaria attenuata (Harman)
- Uncaria canescens (Harman)
- Uncaria orientalis (Harman)
SAPOTACEAE
- Chrysophyllum lacouritianum (Norharman etc.)
SYMPLOCACEAE
- Symplocos racemosa (Harman)
- Fagonia cretica (Harman)
- Peganum harmala (Harmalin, harmin, etc.)
- Tribulus terrestris (Harmin etc.)
- Zaygophyllum fabago (Harmin etc.)
K├żlla: [9]
Om de olika alkaloiderna
Generellt om potensen hos de olika molekylerna:
"In experiments designed to ascertain relative MAO inhibiting abilities, Udenfriend and associates (1958) determined harmine and harmaline to be about equal in activity with harman and tetrahydroharmine less potent, McKenna and coworkers (1984a) obtained similar results, as did Buckholtz & Boggan 1977, but found harmaline to be slightly more active than harmine. In contrast to this is McIsaac & Est├®vez 1966 who reported harman as having the greatest activity (and norharman having yet greater activity). McIsaac & Est├®vez used calf liver mitochondrial homogenates, while Udenfriend and McKenna had both used rat liver homogenates and Buckholtz & Boggan: rat liver and brain homogenate. (Comments adapted from Ott 1994)" [10]
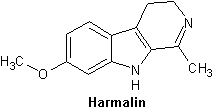
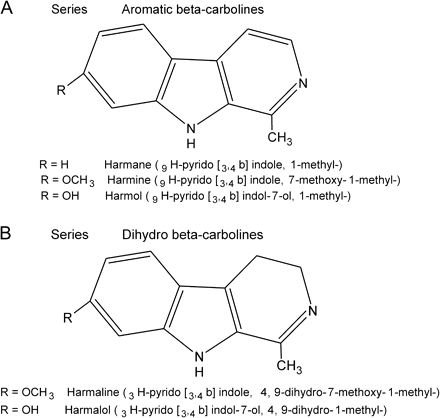
Harmalin
HCl-formnen kristalliserar sig till gula kristaller. Avger ett m├Črkbl├źtt sken under UV-ljus. Harmalin ├żr n├żstan dubbelt s├ź giftigt som harmin, som man kan l├żsa i LD50-tabellen under Kemi.
"Harmaline, like other harmala alkaloids, does not seem to possess classical psychedelic activity (that activity similar to LSD, psilocybin/psilocin or mescaline). Even at high doses (5 mg/kg), the best one can expect from harmaline would be intense nausea, diarrhea, nystagmus and perhaps the sound of rushing water. A 0.5-1.0 mg/kg dose of harmaline (orally) is sufficient to block MAO for 4-6 hours without much of the physiological noise encountered at the higher doses."[11]
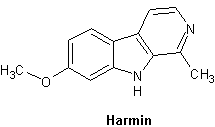
Harmin
Bildar f├żrgl├Čsa rombiska kristaller i metanol. Avger ett m├Črkbl├źtt sken under UV-ljus.
Orally active at 8 mg/ kg; Intravenously at 2 mg/ kg (Naranjo 1967 and Ott 1993.) When smoked, far smaller amounts (even normally trivial) produce a discernible interaction with tryptamines and/or LSD
"120 mg of harmine base (1.5 mg per kilogram is a better view) is usually recommended as the absolute lower limit for full oral activation of DMT. Some Ayahuasca brews have been found to contain slightly in excess of 400 mg per dose."
"Metabolism may vary widely between different species. Half-life in rats is 6 hours. Harmine (and its metabolites) show a 3 hour half-life in humans (however, the half-life of harmine in humans given ayahuasca orally was reported to be less than two hours according to McKenna et al. 1998 Maximum plasma levels were reached 102 minutes after ingestion.) Harmine will noticeably interact with free base tryptamines or orally activate tryptamines for only up to around 4 hours after its ingestion. This refers to harmine pre-administered rather than concurrently taken. Harmaline and tetrahydroharmine are likely to have a slightly longer duration and be handled and processed differently, if observations from other species can be considered an indicator. Detailed human pharmacological data is apparently only now beginning to be generated." [10]
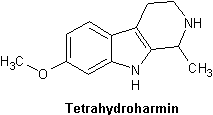
Tetrahydroharmin (THH)
├är b├źde MAO-h├żmmare och svag SSRI. Finns i h├Čg koncentration i Banisteriopsis caapi.
"tetrahydroharmine (THH), the second most abundant ├¤-carboline in the beverage, acts as a weak 5-HT uptake inhibitor and MAOI. Thus, THH may prolong the half-life of DMT by blocking its intraneuronal uptake, and hence, its inactivation by MAO, localized in mitochondria within the neuron. On the other hand, THH may block serotonin uptake into the neuron, resulting in higher levels of 5HT in the synaptic cleft; this 5-HT, in turn, may attenuate the subjective effects of orally ingested DMT by competing with it at post-synaptic receptor sites (Callaway, et al., 1997)." [12]
"Naranjo 1967 reported the racemate to be orally active and 300 mg as equivalent to 100 mg of harmaline. Psychotropic above 3 mg/ kg iv or 12 mg/ kg oral. Ott 1996 citing Naranjo 1967."
"Unlike harmine and harmine, tetrahydroharmine reacts with Ehrlich's reagent. Unlike the tryptamines which react within 30 minutes and develop a dark blue color, THH produces a characteristic robin's egg blue over a 24 hour period. McKenna et al. 1984."
"Tetrahydroharmine is said in the literature to be one third the activity of harmaline but this is based on one single bioassay in one individual. It is not known whether it is able to orally activate DMT and apparently no one has looked into the matter beyond evaluating its relative MAOI properties. MAOI capabilities alone apparently do not guarantee effectiveness at oral activation of DMT. While many MAOIs (including many more dangerous prescription MAOIs) can serve as oral activators; there are also good MAOIs that apparently do not. The evidence provided by some ayahusaca brew compositions suggests THH to be an effective oral activator for DMT despite its poor MAOI stature but apparently formal studies are lacking." [10]
Harman
Lyser bl├źviolett i UV-ljus.
"results suggest that harmane is absorbed into the systemic circulation more completely than harmine. Upon entering the body, harmane can be metabolized to form harmine; the latter may better distribute to the tissue compartment."[13]
Harmol
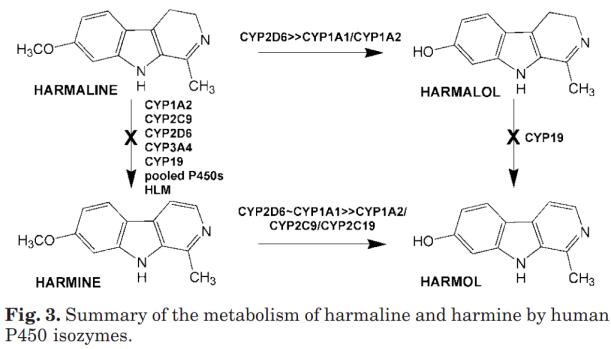
Metabolit fr├źn harmin som O-demetyleras av CYP2D6 i kroppen.
Harmalol
Metabolit fr├źn harmalin som O-demetyleras av CYP2D6 i kroppen. Ostabil i luften.
Kemi
| Namn | Formel | L├źngt namn |
|---|---|---|
| Harmin (banisterin) | C13H12N2O | 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole |
| Harmalin (harmidin) | C13H14N2O | 4,9-Dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole |
| Tetrahydroharmin | C13H16N2O4P | 7-Methoxy-1,2,3,4-Tetrahydro-Harmine |
| Harminsyra | Methyl-7-methoxy-b-carboline-1-carboxylate | |
| Harmilinsyra | 7-methoxy-3,4-dihydro-b-carboline1-carboxylic acid | |
| Harmanamid | 1-carbamoyl-7-methoxy-b-carboline | |
| Acethylnorharnin | 1-acethyl-7-methoxy-b-carboline | |
| Harmalol | C12H12N2O | 11-Hydroxyharmalan |
| Harman | C12H10N2 | 1-methyl-9H-b-carboline |
F├Čr information om l├Čslighet i olika v├żtskor, se Extrahering.
Metabolism
LD50
| Ämne | LD50 |
|---|---|
| Harmin | Dosering under skinnet p├ź r├źttor, 200 mg/kg. |
| Harmalin | Dosering under skinnet p├ź r├źttor, 120 mg/kg, (iv p├ź m├Čss 38mg/kg) |
| Harman | Dosering under skinnet p├ź r├źttor, 200 mg/kg. |
Externa L├żnkar
- Ōåæ United States Patent 5591738 - Method of treating chemical dependency using b-carboline alkaloids, derivatives and salts thereof
- Ōåæ The effects of b-carbolines in rats trained with ibogaine as a discriminative stimulus
- Ōåæ Inhibitory Effect of Harmane on Morphine-Dependent Guinea Pig Ileum
- Ōåæ Norharman and alcohol-dependency in male Wistar rats
- Ōåæ Effects of harman and harmine on naloxone-precipitated withdrawal syndrome in morphine-dependent rats
- Ōåæ High-affinity binding of b-carbolines to imidazoline I2B receptors and MAO-A in rat tissues: Norharman blocks the effect of morphine withdrawal on DOPA/noradrenaline synthesis in the brain
- Ōåæ Dokumentation fr├źn Takiwasi
- Ōåæ Erowid Syrian Rue Vault : Three Beta-Carboline Containing Plants (...). H├żmtad 2011-10-18
- Ōåæ Klipp fr├źn Ayahuasca Analogues
- Ōåæ 10,0 10,1 10,2 Erowid Online Books : "Ayahuasca: alkaloids, plants, and analogs" by Keeper of the Trout
- Ōåæ Tryptamines Beta-Barbolines and You av J.C. Callaway
- Ōåæ Ayahuasca.com
- Ōåæ Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. H├żmtad 2011-10-18
Harmala alkaloid Wikipedia (en)