 |
MAGISKA MOLEKYLERS WIKI |
Flavonoider
Innehåll
[göm]Generell information
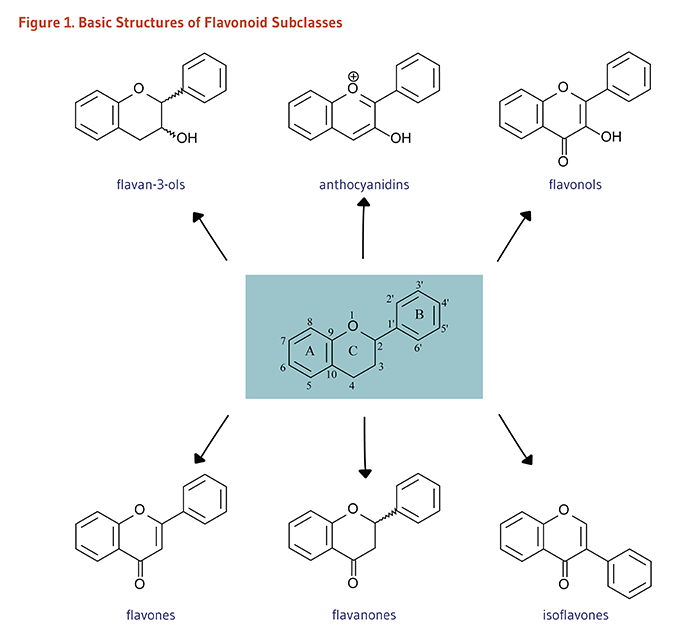
Flavonoider kan delas in flera klasser utifrån deras oxidativa status och substituenter. Psykoaktiva flavonoider tillhör främst klasserna flavoner, flavanoner, isoflavoner, flavonoler, flavanonoler, flavan-3-oler, antocyanidiner och diverse glykosidformer av dessa. Flavan är grundskelettet för alla utom isoflavonerna.
Bildkälla[1]
Flavonoider blev första kända för deras egenskap som färgämnen i växters blad, blommor, frukter och fruktskal[2]. Ordet flavus är latin och betyder gul. Alla flavonoider är dock inte färgade.
Många flavonoider har medicinska användningsområden[3][4][5][6]. Här kan nämnas bl.a antioxidativa, antiproliferativa, antiinflammatoriska, proapoptotiska och kramplösande egenskaper, gynnar kardiovaskulära funktioner, hämmar allergiska reaktioner och astma, cancerhämmande egenskaper. Vissa har även antivirala egenskaper mot exempelvis SARS-virus[7] och förutspås kunna behandla COVID-19[8][9][10].
Upptagningen i kroppen är god, eventuella glykosidgrupper hydrolyseras i kroppen till agkykonerna som lätt passerar blod-hjärnbarriären.
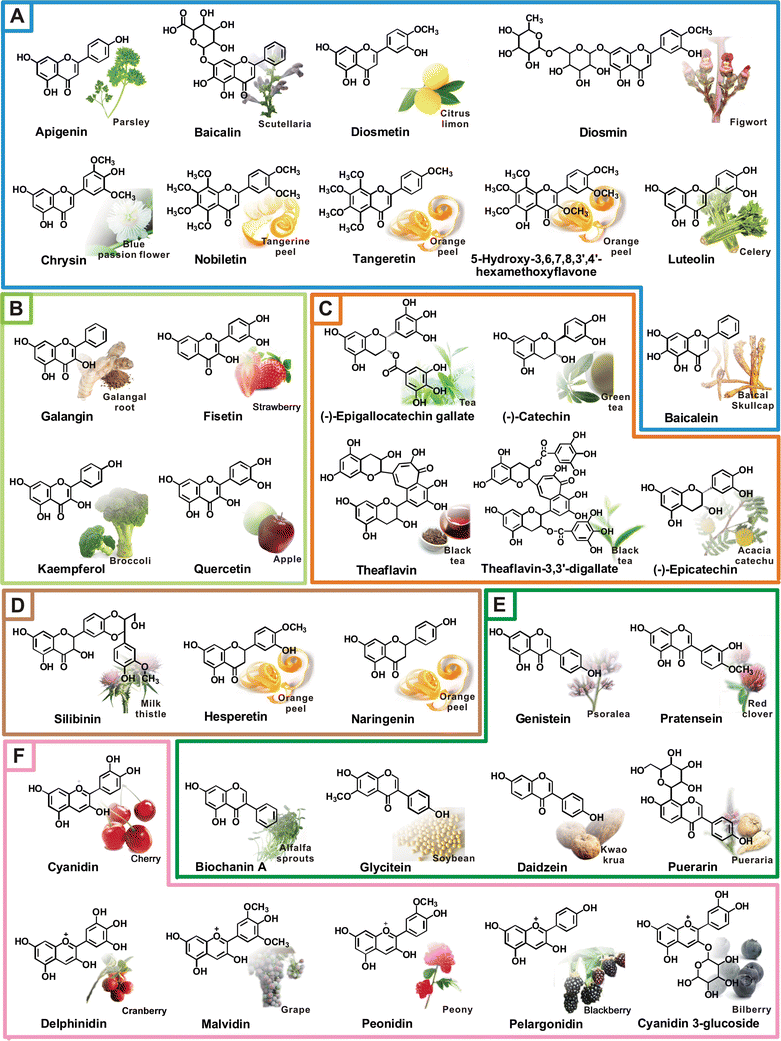
Några av molekylerna som tas upp i denna artikeln:
- A = Flavoner
- B = Flavonoler
- C = Flavan-3-oler (catechiner)
- D = Flavanoner
- E = Isoflavoner
- F = Anthocyanidiner
Bildkälla[11]
Psykoaktiva flavonoider
Det finns mer än 6000 kända flavonoider, men vi skall bara titta närmare på ett fåtal av dessa som kommer från växtvärlden och är psykoaktiva, antingen genom att påverka GABA-, NMDA-, adenosin[12]- eller opioid-receptorer, eller genom att vara MAO-hämmare. Effekterna som vissa flavonoider hade på receptorer som benzodiazepiner binder till upptäcktes av forskare från Argentina på 1990-talet[13] och med åren har fler och fler syntetiska och naturliga molekyler identifierats[4].
Psykoaktiva flavonoider har framför allt anaxolytiska (ångestdämpande) och hypnotiska effekter. Effekten orsakas antingen av MAO-hämning som leder till ökade nivåer av kroppsegna neurotransmittorer såsom serotonin, eller så är det flavonoider som har olika former av påverkan på GABA, som agonister, antagonister eller modulatorer. Här återfinns både flavonoider som verkar snarlikt klassiska bensodiazepiner, andra ger ångestdämpande effekt utan att samtidigt vara sederande, muskelavslappande eller beroendeframkallande. Vissa har t.o.m visat sig kunna förbättra olika kognitiva funktioner.
Det finns en lång rad växter som använts i naturmedicinen sedan urminnes tider för sina avslappnande, ångestdämpande eller sömngivande egenskaper, men de exakta farmakologiska verkningsmekanismerna har inte varit kända. Fram tills för bara 10-20 år sedan har man exempelvis letat efter alkaloider och andra molekyler utan att finna godtagbara förklaringar. Men psykoaktiva flavonoider kan bidra till att förklara varför flertalet av växterna fungerar som de gör. En annan dimension är förståelsen av grönsaker, bär och frukters betydelse i kosten. Vi äter varje dag en viss mängd av några av flavonoiderna i listorna nedan. Inte tillräckliga mängder för att de skall ge stor effekt, men inte heller obetydlig.
Notis gällande IC50-värden i texten och tabellerna: Lägre värde = potentare. IC50 är koncentrationen som behövs för 50% hämning.
| “ | Many investigators have noted structural similarities between certain flavonoids and benzodiazepines, such as diazepam, that are the most widely studied positive modulators of GABAA receptors. Benzodiazepines can act on these receptors via ‘two distinct and separable mechanisms’ (Walters, Hadley, Morris, & Amin, 2000). At nanomolar concentrations, benzodiazepines act in a classic flumazenil-sensitive manner to enhance the action of GABA, while at micromolar concentrations, benzodiazepines act in a flumazenil-insensitivemanner. Flavonoids can act on GABAA receptors at low concentrations in either a flumazenil-sensitive or flumazenil-insensitive manner as modulators of these receptors (Hanrahan et al., 2011). Furthermore, many flavonoids act in a biphasic manner, potentiating GABA actions at low concentrations and inhibiting at high concentrations. In addition, some flavonoids have agonist actions on certain GABA receptors, directly gating the receptor in the absence of GABA. Clearly flavonoids can interact with a variety of specific active sites on ionotropic GABA receptors. Unraveling these active sites remains a major task. — Hanrahan (2015)[14] |
” |
| “ | Linden flowers (Tilia sp. — Tiliaceae) have been used around the world as a tranquiliser, and it has been shown that quercetin- and kaempferol flavonoids are responsible for the sedative effect Heather, (Calluna vulgaris (L.) Hull. — Ericaceae) which traditionally has been usedas a nerve calming remedy, was found to possess MAO-A inhibitory activity, with quercetin being the active component.
... The flavonoid glycosides linarin, 2S-hesperidin, 2S-neohesperidin, 2S-naringenin, diosmin, gossipyn and rutin were found to exert a depressant action on the CNS of mice following i.p. injection, measured in the hole board, thiopental induced sleeping time and locomotor activity tests ... The aglycone apigenin showed no anticonvulsant or anxiolytic properties in vivo. On the other hand, hispidulin, which is 6-methoxyapigenin, had anticonvulsive activity in a model of epilepsy in seizure-prone Mongolian gerbils. ... Chrysin, a flavone from Passiflora coerulea L. (Passifloraceae) increased the number of entries into and time mice spent in the open arms in the elevated plus-maze test of anxiety, consistent with an anxiolytic action. Chrysin also increased the time spent on head-dipping. In contrast, chrysin had no myorelaxant action, indicating that chrysin possesses anxiolytic. The flavone ororylin A isolated from the Chinese medicinal plant Scutellaria baicalensis Georgi (Lamiaceae), which is used for sedation, showed selective sedative and anticonvulsant activity in vivo, which support a subtype specific activity on the GABAA-receptor of the flavone. Epigallocatechin, a trimer of catechin which is found in green tea, had dose-dependent anxiolytic, sedative and amnesiac activity, likely mediated via the GABAA-receptor. The in vivostudies show that flavonoids are able to beabsorbed after oral administration, pass the blood-brain barrier and do have various effects on the CNS. ... A number of monoflavonoids with affinity to the GABAA-benzodiazepine site have been isolated from plants. Apigenin was isolated due to the compound’s affinity to the benzodiazepine site from Matricaria retutica (Asteraceae) [1], Tanacetum parthenium (Asteraceae) [2] and Sersia dentata Thunb. (Anacardiaceae) [38]. A number of Ki-values have been reported: 3 µM [39,40], 4 µM [1], 9 µM [2] and 40 µM [38]. 6-methylapigenin was isolated from the sedative plant Valeriana wallichii D.C. (Valerianaceae), and had a Ki-value 495 nM [41]. Dinatin, skrofulein, cirsilineol and hispidulin were isolated from Artemisia herba-alba Asso (Asteraceae), and IC50-values of 1.3 µM, 23 µM, 104 µM and 8 µM were reported, respectively [42,43]. Chrysin was isolated as the active compound in Passiflora coerulea (Passifloraceae) with a Ki-value of 3 µM [44]. ... Several flavonoids have been identified as inhibitors of MAO-A and MAO-B. The flavonols kaempferol and quercetin and the flavones apigenin and chrysin were isolatedfrom a standardized Gingko biloba extract by means of HPLC [57]. All four flavonoids were identified as MAO-A inhibitors with IC50 values of: kaempferol (0.7 µM), apigenin (1 µM), chrysin (2µM) and quercetin (5 µM). ... Quercetin was isolated from heather (Calluna vulgaris (L.) Hull – Ericaceae) and identified as a MAO-A inhibitor with an IC50 value of 18 µM. In the same assay clorgylin, a selective MAO-A inhibitor, had an IC50 value of 0.2 µM [4]. In another study it was reported that quercetin is a selective MAO-A inhibitor with an IC50 value of 0.01 µM for MAO-A and 20 µM for MAO-B [58]. Quercetrin, isoquercetrin, rutin and quercetin isolated from Melastoma candidum D. Don (Melastomataceae) were shown to inhibit MAO-B with IC50 values of 19, 12, 4, 11 µM, respectively, in an assay where deprenyl (a selective MAO-B inhibitor) had an IC50 value of 19 µM [59]. The flavan-3-ols (+)-catechin and (-)-epicatechin were isolated from Uncaria rhynchophylla (Miq.) Jacks. (Rubiaceae) and found to inhibit MAO-B with IC50 values of 89 and 59 µM, respectively, while deprenyl had an IC50 value of 0.3 µM [60]. Two flavonoids isolated from Sophora flavescens Ait. (Fabaceae) exhibited monoamineoxidase inhibitory activity: formononetin an isoflavone with IC50 values of 21 µM (MAO-A) and 11 µM for MAO-B and the flavanone kushenol F with IC50 values of 104 µM (MAO-A) and 63 µM for MAO-B [61]. Naringenin was isolated from Mentha aquatica L. (Lamiaceae) in a bioassay-guided fractionation process [62]. The IC50 value for MAO-A inhibition was 955 µM and 288 µM for MAO-B in an assay where the IC50 value of clorgylin was 0.0003 µM and 0.1 µM for deprenyl. In a recent study the inhibitory effects of pure anthocyanidins on MAO-A and MAO-B activity was investsigated [63]. The following IC50 values were obtained for MAO-A and MAO-B inhibitory activity, respectively: malvidin (22 µM and 19 µM), pelargonidin (27 µM and 43 µM), cyanidin (30 µM and 32 µM), peonidin (31 µM and 22 µM), petunidin (32 µM and 43 µM), delphinidin (35 µM and 31 µM). In the same study different glycosides and diglycosides of the above mentioned anthocyanidins were also studied with IC50 values in the range of 29-117 µM for MAO-A inhibition and 31-242 µM for MAO-B inhibition. All of the active flavonoids identified in the above possess inhibitory activity on MAO-A, MAO-B or both. Furthermore, this inhibitory activity is not confined to a single flavonoid class as all the
classes presented in figure 1 are represented. |
” |
| “ | 2.3.4 Interactions between Flavonoids and Monoamine Oxidase
A lot of research has been done using plants phytochemicals in the investigation of the inhibitory effects for both MAO-A and B. A number of flavonoids have been isolated and identified as inhibitors of both the isoforms. These include flavonols such as kaempferol (12), quercetin (10) and flavones; apigenin (8) and chrysin (31) from Gingko biloba (Ginkgoaceae) extract (Sloley et al., 2000). MAO-A inhibition activities from these flavonoids in this study were as follows with IC50 values: chrysin (2µM), apigenin (1 µM), kaempferol (0.7 µM) and quercetin (5 µM) in which phenelzine (60) the standard was (0.04 µM). Quercetin (10) from (Calluna vulgaris (Ericaceae) demonstrated MAO-A inhibitory activity of IC50 18 µM in a study by Saaby et al (2009) in which clorgylin (32) an irreversible and a selective MAO-A inhibitor, used as the standard showed IC50 0.2 µM. Chimenti et al., 2006 in a study reported quercetin (10) showing high inhibition activity towards MAO-A, IC50 0.01 µM while inhibition activity for MAO-B was IC50 20 µM. Quercetrin-3-glucoside (isoquercetrin) (67), rutin (68) and quercetin (10) isolated from Melastoma candidum (Melastomataceae) also inhibited MAO-B with IC50 19, 12, 4, 11 µM respectively in which selegiline (55), used as the standard had IC50 19 µM (Lee et al., 2001). The flavan-3-ols, (+)-catechin-20 and (-)-epicatechin-22 also from Uncaria rhynchophylla (Rubiaceae) showed MAO-B activity of IC50 89 and 59 µM respectively where IC50 for selegiline (55) was 0.3 µM as reported by Hou et al (2005). Formononetin (69), an isoflavone isolated from the roots of Sophora flavescens (Fabaceae), showed MAO-A inhibitory activity with an IC50 21 µM and 11 µM for MAO B. Kushenol (F)-(70) also isolated from the same plant exhibited IC50 104 µM (MAO-A) and 63 µM (MAO-B) according to the report published by Hwang et al (2005). Naringenin (14) from Mentha aquatic (Lamiaceae) (Olsen et al., 2008) had inhibition of IC50 955 µM (MAO-A) and 288 µM (MAO-B) while C50 value for clorgylin was 0.003 µM and deprenyl showed IC50 0.1 µM. On the basis of the reported literature of the ability of naringenin in crossing the blood brain barrier, they concluded that the isolated compound can enter into the CNS and be used in the treatment of depression-like conditions (Youdim et al.,2004). Investigations of pure anthocyanidins inhibitory activity on MAO-A and B have also been done (Dreiseitel et al., 2009) in which IC50 values were determined: delphinidin-3 (35 µM and 31 µM) malvidin-4 (22 µM and 19 µM), cyanidin-2 (30 µM and 32 µM), pelargonidin-5 (27 µM and 43 µM), petunidin-7 (32 µM and 43 µM) and peonidin-6 (31 µM and 22 µM). The same study also conducted the inhibitory activities of different glycosides and diglycosides of the anthocyanidins and IC50 ranged from 29-117 µM for MAO-A and 31-242 µM for MAO-B. Han et al (1987) isolated flavonoids such as apigenin (8), eriodictyol (16) acacetin (71), luteolin (9) and diosmetin (72) from Chrysanthemum indicum and screened them for their MAO B inhibitory activities. The research revealed that acacetin (71) and diosmetin (72) showed good inhibitory activity towards rat liver mitochondrial monoamine oxidase MAO-B with an IC50 value of 2.46 and 2.11mM respectively. A study by Ryu et al (1988) led to the isolation of apigenin (8) and kaempferol (12) from Sophorae flos (Fabaceae) and the inhibitory effects towards rat brain mitochondrial monoamine oxidase MAO-A with an IC50 value of 10µM each. This research revealed that both compounds did not inhibit MAO-B. Haraguchi et al (2004) isolated 5-hydroxyflavanone from Gentiana lutea which showed an IC50 value of 39.6 and 3.8 µM towards both rat brain mitochondrial monoamine oxidase MAO-A and B. A study conducted by Han et al (2007) on Cayratia japonica (vitaceae) led to the isolation of flavonoids from the plant and the compounds were tested for their MAO-A and the inhibitory potency against was: flavone> flavonol> flavone glycoside> flavanonol. According to the series given, apigenin (8) showed inhibitory effects of IC50 1.17µM. They also observed that the level of MAO inhibitory activity decreased as the number of OH groups increased in ring B of the flavone |
” |
Flavoner
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| 6-metylapigenin | Valeriana wallichii | N/A | GABA-A[17][18] |
| Acacetin | Robinia pseudoacacia (robinia), Turnera diffusa (damiana), Betula pendula (vårtbjörk) | 121nM MAO-A, 49 nM MAO-B[19] | MAO-A, MAO-B[16] |
| Apigenin | Matricaria recutita (kamomill), Tanacetum parthenium (mattram), Leonurus cardiaca, Passiflora incarnata, Searsia dentata, Petroselinum crispum (persilja) | 1 µM MAO-A[15], 10 µM NMDA[20] | MAO-A, GABA-A[13][21], NMDA-antagoist. Ångestdämpande hos möss vid 3mg/kg[22][6] |
| Baicalin | Scutellaria lateriflora, Scutellaria galericulata (frossört) | N/A | GABA-A[23][24] |
| Chrysin | Passiflora caerulea | 2 µM MAO-A[15] | GABA-A[25], MAO-A/MAO-B[26][27] |
| Cirsilineol | Artemisia herba-alba | 104 µM GABA-A[15] | GABA-A |
| Cirsimaritin | Salvia triloba | N/A | GABA-A[28][29]. Även adenosinantagonist[30] |
| Diosmetin | Citrus limon (citron), Vicia (vicker) | 2.11 µM MAO-B[31] | MAO-B[16] |
| Hispidulin (= Dinatin) | Artemisia herba-alba, Salvia officinalis (kryddsalvia) | 1.3 µM GABA-A[13] [15] | GABA-A[32][33] |
| Kushenol F | Sophora flavescens | 104 µM MAO-A, 63 µM MAO-B[15] | MAO-A, MAO-B[34] |
| Luteolin | Jordnötsskal[35], Artemisia afra[36][37]. Kryddväxter, ex.vis sellerifrön, oregano, enbär | 4.9µM MAO-A[21] | GABA-A[38], även smärtstillande effekter[39] samt hämmare av MAO-A[40]. |
| Salvigenin | Salvia triloba, Rosmarinus officinalis (rosmarin) | N/A | GABA-A[28][29] |
| Skrofulein | Artemisia herba-alba | 23 µM GABA-A[15] | GABA-A |
| Wogonin | Scutellaria baicalensis | N/A | GABA. Ångestdämpande effekt hos möss oralt 7.5-30 mg/kg[41][13] |
Flavonoler och dess glykosider
Den vanligaste typen av flavonoider är flavonolerna som finns i många växters blad och andra pigment.
Quercetin är en potent MAO-hämmare som dessutom är mycket vanligt förekommande. Kapris är en mycket bra källa då den innehåller 234mg/100g quercetin[42] och därtill 259mg/100g kaempferol. Kapris i konservburk innehåller 173mg/100g respektive 131mg/100g. Lökblad verkar också vara väldigt lovande[43].
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| Kaempferol | Gingko biloba, P. caerulea, P. incarnata, Tilia (lind), Crocus sativus (saffran) | 0.7 µM MAO-A[15] | MAO-A och MAO-B[16] |
| Quercetin | Quercus robur (ek), Oenothera biennis (nattljus), Capparis spinosa (kapris), Allium cepa (gul lök), Hypericum perforatum (johannesört), Camellia sinensis (te), Calluna vulgaris (ljung), Levisticum officinale (libbsticka) | 0.01-18 µM MAO-A, 20 µM MAO-B[15] | Främst MAO-A, men även svag MAO-B[16][40][44][45][21]. GABA[46]. Antidepressiv effekt[47][48][5] |
| Quercetrin, isoquercetrin | N/A | 19 µM / 12 µM MAO-A[15] | MAO-A, MAO-B[16]. Glykosid av quercetin. |
Flavan-3-oler / catechiner
Återfinns i exempelvis te, kakao, äpplen, bär och vindruvor. Främst vitt te och tefrukter är potenta[42], men vanlig mörk choklad är också en bra källa till (-)-epicatechin[49].
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| Catechin & epicatechin | Uncaria rhynchophylla, Camellia sinensis (te), Theobroma cacao (kakao), Acacia catechu | 89 µM / 59 µM MAO-B[15], 35µM[50] | MAO-A, MAO-B[51][52] |
Isoflavoner
Isoflavoner finns främst representerade i familjen Leguminosae, dock inte de psykoaktiva som hör till Fabaceae.
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| Formononetin | Sophora flavescens | 21 µM MAO-A, 11 µM MAO-B[16] | MAO-A, MAO-B[34] |
| Genistein | Psoralea Corylifolia | N/A | MAO-B[53] |
Anthocyanidiner
Anthocyanidiner är aglykoner (saknar glukosidgruppen) av anthocyaniner. Anthocyaniner och Anthocyanidiner står bakom de starka färgena hos många bär och frukter, t.ex blåbär, hallon, jordgubbar, svart vinbär, vindruvor, men återfinns även i olika röda lökar och andra starkt färgade grönsaker[54].
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| Cyanidin | Röda och blå bär såsom blåbär, hallon, svart vinbär, fläder, Aronia. Även rödkål, rödlök, rädisa | 30 µM MAO-A, 32 µM MAO-B[15] | MAO-A, MAO-B[16][55]. Kan användas som pH-indikator. |
| Delphinidin | Viola (violer), Delphinium (riddarsporrar), blåbär, svart vinbär | 35 µM MAO-A, 31 µM MAO-B[15] | MAO-A, MAO-B[16] |
| Malvidin | Primula (vivor), Vitis vinifera (vindruvor), blåbär, odon[56] | 22 µM MAO-A, 19 µM MAO-B[15] | MAO-A, MAO-B[16] |
| Pelargonidin | Geranium, (nävor), hallon, jordgubbar, tranbär, granatäpple | 27 µM MAO-A, 43 µM MAO-B[15] | MAO-A, MAO-B |
| Peonidin | Paeonia (pioner), tranbär, plommon, vindruvor, körsbär, blåbär | 31 µM MAO-A, 22 µM MAO-B[15] | MAO-A, MAO-B. 42 mg/100g i tranbär[57] |
| Petunidin | Aronia, Amelanchier alnifolia, vindruvor, "svarta" tomater, blåbär | 32 µM MAO-A, 43 µM MAO-B[15] | MAO-A, MAO-B |
En studie på människor från 2015 har visat att anthocyaniner/anthocyanidiner i svart vinbärsjuice hämmar MAO-B och ger kognitiva förbättringar[58]. Bärens anthocyaniner består till 97% glukosider av delphinidin och cyanidin[59]. Men det är förmodligen inte enbart anthocyanidiner som ger effekten, det finns även gott om quercetin i vinbären.
Att svarta vinbär kan användas som MAO-hämmare har lett till experiment med en ayahuasca-analog som fått namnet "Gummihuasca"[60] (referens till bumbibjörnarnas bumbibärssaft som ger magiska krafter) som ett antal personer haft positiva effekter med. Den har främst kombinerats med psilocybinsvampar.
En patentansökan från 2002 ger oss detaljer om doseringen och tidskurvan. Den är f.ö även intressant att läsa eftersom mycket text handlar om kognitiva förbättringar av intaget av svart vinbärsjuice eller extrakt.
| “ |
Test 2--Demonstration of MAO-Inhibiting Action in Humans In a test in humans, three healthy subjects received 5, 20, and 50 g of a concentrate of black currant juice in 5.5-fold concentration by the oral route. Blood samples were taken at times--15, 0, 30, 60 and 180 minutes. After centrifugation, platelet-rich plasma was obtained, the MAO type B activity in thrombocytes being determined by a method similar to that described for Test 1. Result MAO type B activity is inhibited in all three subjects with dependency upon dosage and time. Maximum inhibition is obtained 60 minutes after application, and is between 70 and 90% at optimum dosage of 20 grams. This result was confirmed by 92% inhibition in the controlled test as specified under Test 5, using 50 g juice concentrate. |
” |
Övriga (flavanoner/flavonglykosider/chalkoner m.m)
Flavanoner återfinns många frukter och örter. I citrusfrukter är vissa rikligt förekommande.
| “ | Considering the sedative effects obtained and the doses injected the activity of the glycosides assayed can be expressed by the following sequence, in decreasing order: 2S-hesperidin > linarin > rutin > diosmin ~ 2S-neohesperidin > gossypin > 2S-naringin.
... We found that, 2S-neohesperidin, 2S-naringin, diosmin, rutin and gossypin, increased the thiopental-induced sleeping time in mice (Table 2), reduced the exploratory parameters in the hole board test (Fig. 1) and also reduced the spontaneous locomotor activity (Table 2). These results indicate that all the glycosides assayed cause a general inhibition of neuronal activity in the CNS. ... Hesperidin properties have been explored in many clinical and experimental conditions (Garg et al., 2001). Nevertheless, its remarkable activity on the CNS eluded detection. This failure may be partially explained by the fact that the drug generally used was the racemic variety provided by the citrus industry, and we found that the CNS active compound is the 2S-(-) isomer (Marder et al., 2003). ... Flavonoids glycosides are easily metabolized by the organism and it could be possible that secondary metabolites may activate GABA-A receptors to mediate sedative effects. However, picrotoxin, a non-competitive antagonist, was unable to block the sedative actions of 2S-hesperidin in vivo at the doses and conditions used. All the data discussed up to here strongly suggest that the CNS depressant action of flavonoid glycosides does not involve classical GABA-A receptors, at least not directly. |
” |
| Namn | Återfinns i | IC50 | Effekt/kommentar |
|---|---|---|---|
| Hesperidin | Apelsinskal, pomerans, pepparmynta | N/A | Centraldepressant[15][6] |
| Agathisflavone | Searsia pyroides | N/A | GABA-A[63] |
| Amentoflavone | Searsia pyroides, Ginko biloba | N/A | GABA-A[63][6] |
| Glabrol | Glycyrrhiza glabra (lakritsrot) | N/A | GABA-A[64] |
| Gossypin | Hibiscus vitifolius | N/A | GABA. 1 mg/kg gav ångestdämpande effekt och 30 mg/kg sederande effekt[65] |
| Isoliquiritigenin | Glycyrrhiza uralensis, Allium ascalonicum (schalottenlök), Glycine max (sojaböna) | 0.453 µM GABA-A[4] | hypnotiska effekter[66] |
| Linarin | Tilia cordata (lindblommor), Menta (myntor) | N/A | Sederande egenskaper[15][67][68] är även en acetylkolinesterashämmare[69] och kan förstärkas med peppar. |
| Naringenin | Mentha aquatica, citrusfrukter | 955 µM MAO-A, 288 µM MAO-B[15] | MAO-A, MAO-B[70], låg aktivitet på GABA. |
| Ororylin A | Scuttelaria baicalensis | N/A | GABA[15] |
| Rutin | Acacia polyacantha spp. camplyacantha, Melastoma candidum | 4 µM MAO-A[15] | MAO-A. Glykosid av quercetin. |
| Vitexin | Passiflora, Acacia senegal | N/A | opioidreceptorer och GABA-A[71] |
- ↑ Linus Pauling Institute: Flavonoids
- ↑ Natural products wiki: Flavonoids
- ↑ Wikipedia: Flavonoid (en)
- ↑ 4,0 4,1 4,2 Flavonoids as GABAA receptor ligands: the whole story? (Wasowski, 2012)
- ↑ 5,0 5,1 Alternative Medicine Review: Quercetin
- ↑ 6,0 6,1 6,2 6,3 Modulation of Ionotropic GABA Receptors by Natural Products of Plant Origin (Johnston, 2006)
- ↑ Small Molecules Blocking the Entry of Severe Acute Respiratory Syndrome Coronavirus into Host Cells (Yi, 2004)
- ↑ Potential Inhibitor of COVID-19 Main Protease (Mpro) from Several Medicinal Plant Compounds by Molecular Docking Study (Khaerunnisa, 2020)
- ↑ Inhibition of SARS-CoV 3CL protease by flavonoids (Jo, 2020
- ↑ On the Inhibition of COVID-19 Protease by Indian Herbal Plants: An In Silico Investigation (Kumar Srivastava, 2020
- ↑ Anti-inflammatory activity of natural dietary flavonoids (Pan, 2010)
- ↑ Interactions Of Flavones And Other Phytochemicals With Adenosine Receptors (Jacobson, 2012)
- ↑ 13,0 13,1 13,2 13,3 Flavonoid modulation of GABAA receptors (Hanrahan, 2011)
- ↑ Interactions of Flavonoids with Ionotropic GABA Receptors (Hanrahan, 2015)
- ↑ 15,00 15,01 15,02 15,03 15,04 15,05 15,06 15,07 15,08 15,09 15,10 15,11 15,12 15,13 15,14 15,15 15,16 15,17 15,18 15,19 15,20 15,21 Flavonoids and the CNS. (Jäger & Saaby, 2011)
- ↑ 16,0 16,1 16,2 16,3 16,4 16,5 16,6 16,7 16,8 16,9 Anti-Oxidant And Monoamine Oxidase Inhibition Studies On The Surface Exudates Of Gardenia Ternifolia (Awas, 2010)
- ↑ Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABA(A) receptors, from Valeriana wallichii. (Wasowski, 2002)
- ↑ 6-methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. (Marder, 2003)
- ↑ Isolation of Acacetin from Calea urticifolia with Inhibitory Properties against Human Monoamine Oxidase-A and -B. (Chaurasiya, 2016)
- ↑ Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. (Losi, 2004)
- ↑ 21,0 21,1 21,2 Monoamine oxidase inhibitory components from Cayratia japonica. (Han, 2007)
- ↑ Overview Flavonoids: A New Family of Benzodiazepine Receptor Ligands (Medina, 1996)
- ↑ GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin. (Wang, 2008)
- ↑ Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. (Liao, 2003)
- ↑ Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. (Brown, 2007)
- ↑ Multifunction of Chrysin in Parkinson's Model: Anti-Neuronal Apoptosis, Neuroprotection via Activation of MEF2D, and Inhibition of Monoamine Oxidase-B. (Guo, 2016)
- ↑ Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. (Wolfman, 1994)
- ↑ 28,0 28,1 GABAA Receptor Modulation by Compounds Isolated from Salvia triloba L. (Abdelhalim, 2014)
- ↑ 29,0 29,1 Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. (Abdelhalim, 2015)
- ↑ Cirsimarin and cirsimaritin, flavonoids of Microtea debilis (Phytolaccaceae) with adenosine antagonistic properties in rats: leads for new therapeutics in acute renal failure. (Hasrat, 1997)
- ↑ Studies on the monoamine oxidase inhibitors of medicinal plants I. Isolation of MAO-B inhibitors from Chrysanthemum indicum (Han, 1987)
- ↑ The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects (Kavvadias, 2004)
- ↑ Flavonoids: some of the wisdom of sage? (Johnston, 2004)
- ↑ 34,0 34,1 Monoamine oxidase inhibitory components from the roots of Sophora flavescens. (Hwang, 2005)
- ↑ Evaluation of luteolin from shells of Korean peanut cultivars for industrial utilization (Radhakrishnan, 2013)
- ↑ Artemisia afra and luteolin
- ↑ The design, preparation and evaluation of Artemisia afra and placebos in tea bag dosage form suitable for use in clinical trials (Dube, 2006)
- ↑ Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. (de la Peña, 2014)
- ↑ Investigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male Sprague-Dawley rat. (Raines, 2009)
- ↑ 40,0 40,1 Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells (Bandaruk, 2014)
- ↑ Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. (Hui, 2002)
- ↑ 42,0 42,1 USDA Database for the Flavonoid Content of Selected Foods, Release 3 (2011)
- ↑ Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. (Miean, 2001)
- ↑ Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. (Yoshino, 2011)
- ↑ MAO-A inhibitory activity of quercetin from Calluna vulgaris (L.) Hull. (Saaby, 2009)
- ↑ Quercetin: further investigation of its antinociceptive properties and mechanisms of action. (Filho, 2008)
- ↑ Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. (Anjaneyulu, 2003)
- ↑ Anxiolytic and sedative-like effects of flavonoids from Tilia americanavar. mexicana: GABAergic and serotonergic participation (Aguirre-Hernández, 2016)
- ↑ Magiska Molekyler: Kakao, Ny partydrog
- ↑ Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson's disease. (Samoylenko, 2010)
- ↑ Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. (Hou, 2005)
- ↑ Monoamin eoxidase inhibitory activity of green tea produced in China, its processing and provincial differences (Chung)
- ↑ Bavachinin and Genistein, Two Novel Human Monoamine Oxidase-B (MAO-B) Inhibitors in the Psoralea Corylifolia Seeds (Zarmouh, 2015)
- ↑ Wikipedia: Anthocyanidin
- ↑ Berry anthocyanins and their Aglycons inhibit monoamine oxidases A and B. (Dreiseitel, 2009)
- ↑ Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland. (Lätti, 2010)
- ↑ Wikipedia: Peonidin (en)
- ↑ Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults (Watson, 2015)
- ↑ Anthocyanins from black currants (Ribes nigrum L.). (Slimestad, 2002)
- ↑ Shroomery: Gummihuasca experiment - Black Currant Juice to increase mushroom effects
- ↑ US Patent 5262162: Cerebral-Activating Compositions
- ↑ Central nervous system depressant action of flavonoid glycosides (Fernández, 2006)
- ↑ 63,0 63,1 Biflavones from Rhus species with affinity for the GABAA/ benzodiazepine receptor (Svenningsen, 2006)
- ↑ Hypnotic effects and GABAergic mechanism of licorice (Glycyrrhiza glabra) ethanol extract and its major flavonoid constituent glabrol. (Cho, 2012)
- ↑ The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. (Fernandez, 2009)
- ↑ Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. (Cho, 2011)
- ↑ Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis (Fernández, 2004)
- ↑ Effects of Piperine on the Intestinal Permeability and Pharmacokinetics of Linarin in Rats (Feng, 2014)
- ↑ Linarin, a selective acetylcholinesterase inhibitor from Mentha arvensis. (Oinonen, 2006)
- ↑ Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. (Olsen, 2008)
- ↑ Antinociceptive effects of vitexin in a mouse model of postoperative pain (Qing, 2016)