 |
MAGISKA MOLEKYLERS WIKI |
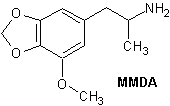
MMDA
Generell information
MMDA kan skapas genom aminering av den eteriska oljan myristicin.
Claudio Naranjo skriver i boken "The Healing Journey" om sin forkning om MMDA som verktyg i psykedelisk terapi. MMDA besitter ├Čnskv├żrda egenskaper f├Čr detta ├żndam├źlet. [1].
MMDA ├żr narkotikaklassad i Sverige.
Effekt / dosering
Effekten liknar inte den som "kusinerna" MDA och MDMA ger. MMDA ger mindre stimulering, mera CEV:s och effekten ├żr dr├Čmlik.
Oral dosering: 100 - 250 mg
"(Om muskotolja:) The substance myristicin is the major component of this fraction, and if it were to undergo the transformation parallel to that proposed for elemicin, a second amphetamine, 3-methoxy-4,5-methylenedioxy amphetamine (MMDA), would result."
"This latter amphetamine has been synthesized from myristicin in vitro, by a three-step process. trans-Isomyristicin, obtained from myristicin by heating in alcoholic potassium hydroxide, is converted by means of tetranitromethane to ├¤-nitro isomyristicin. Reduction of this nitropropene with LiAlH4 led smoothly to MMDA, which was isolated as the hydrochloride. Examination of MMDA in mice and dogs displayed a behavioural and toxicological pattern similar to that of TMA, both quantitatively and qualitatively. Preliminary human evaluation revealed initial intoxication at about 1.0 mg/kg (as free base). More extensive assay has confirmed a thoroughly effective hypnogogic dementia at less than 2.0 mg/kg. The psychotomimetic syndrome was complete at this level: increased dosages (to 3.2 mg/kg) merely prolonged the duration of the episode. Thus the potency of MMDA is somewhat greater than TMA and nearly three times greater than mescaline." K├żlla: [2]
Kemi
| Systematiskt namn | 3-metoxi-4,5-metylendioxyamfetamin | |
| Trivialnamn | MMDA | |
| Kemisk formel | C11H15NO3 | |
| Sm├żltpunkt | 133-134 ┬░C (HCl) | |
| Kokpunkt | ||
| Molmassa | 209.2417 g/mol | |
| Densitet | ||
| L├Čslighet | L├żs under extrahering |
LD50 ligger n├źgonstna runt 100mg/kg [3]
Isomer:
- MMDA-2 (2-Metoxi-4,5-metylendioxyamfetamin
- MMDA-3a (2-Metoxi-3,4-metylendioxyamfetamin
- MMDA-3b (4-Metoxi-2,3-metylendioxyamfetamin
Externa l├żnkar
Sidan ├żndrades senast 11 april 2009 klockan 04.22.
Den h├żr sidan har visats 7┬Ā345 g├źnger.