 |
MAGISKA MOLEKYLERS WIKI |
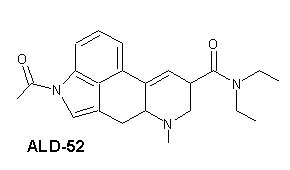
ALD-52
Namn: Känd i USA i slutet av 60-talet under namnet "Sunshine" eller "Orange Sunshine", läs mer nedan.
Generell information
ALD-52 är en ergotalkaloid och LSD-analog med liknande potens och effekter. Men som Alexander Shulgin förklarar i citatet nedan finns det även en del subjektiva uppfattningar om skillnader:
| “ | This material has been explored in the 50-175 microgram range and there are a number of human trials reported, with varying conclusions. One found that there was less visual distortion than with LSD and it seems to produce less anxiety and was somewhat less potent than LSD. Another report claimed it was more effective in increasing blood pressure. Yet another could not tell them apart. — TiHKAL av A. Shulgin[1] |
” |
ALD-52 påstods ha sålts i Kalifornien under namnet "Orange sunshine" i slutet av 1960-talet. En kemist stod åtalad för att ha tillverkat LSD försökte använda ALD-52 som försvar i en rättegång genom att hävda att kemisten tillverkat den lagliga drogen ALD-52 som fallit sönder till LSD i polisens förvar/provtagningsförfarande. 2017 kom det fram att kemisten som åkte fast hela tiden hade tillverkat LSD och historien om ALD-52 var fabricerad[2]
Det finns uppgifter som gör gällande att ALD-52 omvandlas till LSD i kroppen:
| “ | Monroe and Heath (1961) compared activity of several LSD analogs in monkeys and cats. LSD was the most active. DAM and LPD (see Table 6) produced moderate EEG activity and about 10% of LSD's psychological activity. MLD-4 and LSM had minimal effects on the EEG and 20-40% of LSD's psychological activity. l-LSD, BOL, and UML were inactive. In sharp contrast ALD-52 was as active psychologically as LSD but produced minimal EEG changes. This could be due to the slow release of LSD from its acetyl derivative by hydrolysis in the body. — Hallucinogens (1967)[3] |
” |
LSD-analogen 1P-LSD har visat sig omvandlas till LSD i mänskligt blodserum.[4][5] Det bör ge en hög sannolikhet att ALD-52 genomgår samma hydrolys på 1-positionen även om det inte bevisats. Den kände kemisten David Nichols håller med:
| “ | It is well known in organic chemistry that N-acyl indoles of all kinds hydrolyze easily. Whether or not it hydrolyzes in the body has not been tested. Taken orally, the low pH of the stomach would likely take it off readily. At some point, you have to accept that some mechanisms in organic chemistry are accepted, although the exact experiment appears not to have been done on ALD-52.
.... I don’t think any mysticism is involved. It is a simple extrapolation from de-acetylation of all indoles under mild conditions, to deacetylation of ALD-52 in vivo. From what we now know about the structure-activity relationships of LSD analogues, the N(1)-acetyl compound would not be active. If it is active in man, it is only by hydrolysis of the acetyl group to give LSD. |
” |
Kemi
| Systematiskt namn | 1-Acetyl-N,N-diethyllysergamide | |
| Trivialnamn | ALD-52 | |
| Kemisk formel | C22H27N3O2 | |
| Smältpunkt | ||
| Kokpunkt | ||
| Molmassa | 367,48 g/mol | |
| Densitet | 1.20 +/- 0.1 g/ml | |
| Löslighet | Läs under extrahering |
Refraktivt index (uppskattat): 1.619 +/- 0.05
Ytspänning (uppskattat): 43.0 +/- 7.0 dyne/cm
Externa länkar
- ↑ TiHKAL #26. LSD-25
- ↑ Erowid: Tim Scully Describes Failed ALD-52 Legal Gambit
- ↑ The Hallucinogens (1967) Av Abram Hoffer & Humphry Osmond
- ↑ Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD) (Brandt, 2015)
- ↑ Pharmacokinetics and subjective effects of 1P‐LSD in humans after oral and intravenous administration (Grumann, 2020)
- ↑ Bluelight: The Big & Dandy 1P-LSD Thread, Volume 1
Magiska Molekyler: ALD-52, kontrollerad substans?, Hjälp mig reda ut det här.
Magiska Molekyler: ALD-52, eller Min Käre Vän Orange Sunshine, Om LSD-analogen ALD-52.